Return to Sitemap
2006-11-06 Rev. 2008-03-29, 2009-08-27, 2010-03-27
![]()
| My purpose in building this page is to think about how the handling of glass on the pipe might be simulated on a computer, mostly for curiosity, I guess. As becomes clear in the notes, this is not easy and I may never get close to a solution 2010-03-27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Glass Simulation Viscosity My current projects are ambitious dreams and frustrations. When I went to look at the online virtual world, Second Life, I could not even go and try it because it didn't like my otherwise competent video card. When I look at your product, I can't even get a good list of what you do include. The actual "project" is to simulate the handling of molten glass being taken out of a pool of glass with a pipe and being turned to keep it from dripping and the changing handling with cooling temp and increasing viscosity. The turning would have to be maintained, perhaps with repeated use of a specific key on the keyboard while steering the pipe, perhaps with the up-down-left-right cursor keys. If that were made to work, then a second project might be to inflate a small bubble within the glass and keep it centered while further inflating. More complicated would be to add molten glass to the outside to permit further inflation without getting too thin. I haven't done any programming on this, although based on my experience and education I could do it with a substantial increase of my knowledge of viscosity physics. Mike Firth Furnace Glass Blowing Web Site MF Notes: Possible modeling Gathering  Crude model: starting viscosity, increasing value with time, flow and shear
formulas Crude model: starting viscosity, increasing value with time, flow and shear
formulasPrime model: viscosity based on temperature, therefore temp of furnace (and pipe?), with decreasing temp a function of radiant and convective loss of heat. Bubble and Centering  Marvering, blowing, starting bubble or not based on viscosity and internal and
surface temp. Marvering, blowing, starting bubble or not based on viscosity and internal and
surface temp.Regathering and inflating Regathering, amount of glass and over or okay heating with collapse Centering and inflating with wall thickness. I am just beginning to explore whether I am capable of modeling a simulation of the handling of molten glass as in glassblowing. In looking at your page http://www.plamedia.co.jp/english/soft_x/sundybasic.html I am somewhat overwhelmed by what I have to learn in spite of a background in engineering and a number of years as a computer programmer. The model for gathering glass is going to be a rotating cylinder (the end of the pipe) turning at a few rpm in a liquid with a beginning viscosity about like that of honey (roughly 3000-6000 cP). In the liquid glass, the temperature of the end of the pipe is a dull red heat at the start and the glass is about 2050F. The pipe rapidly increases in temperature while the glass near the pipe is cooled. The pipe is rotated to gather glass around it and then is removed from the pool of glass. The pipe must be kept rotating to keep the glass from dripping off and to keep it evenly distributed. Does anything in your models come close to dealing with this kind of situation? For the record, the next step in my model will be to deal with the change in viscosity as the temperature of the glass rapidly falls, the effect of marvering (rolling the cylinder of glass on a steel surface to chill the glass surface), and blowing a bubble inside the still hot core. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
http://www.soe.rutgers.edu/knight/CCD/si_2000/cheng_xu.htm "The temperature lag between the surface and centerline temperature, defined as Tlag=(Ts-Tc)/Tc, describes the thermal response process in the glass. It is found that the heating zone length affects the lag level only slightly. However, the influence of draw speed is significant. The temperature difference between the surface and centerline temperature increases about 30% when the draw speed goes up from 15 m/s to 25 m/s. This result suggests that the residence time of the glass rod inside the heating furnace is the dominating factor to control the radial temperature difference, since a longer residence time causes the preform to be more uniformly heated. The radial non-uniformity in the temperature in the perform noticeably affects the velocity field, because glass viscosity is an exponential function of the temperature. This can cause the redistribution of the material dopants and impurities, and therefore impact the fiber quality. Similarly, the axial velocity lag between the centerline and the surface in the neck-down region is defined as vlag=(vs-vc)/vc. The velocity lag is very sensitive to the furnace dimensions. The lag decreases significantly with an increase in the furnace length."
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
[http://www.ftimeters.com/pages/uvc.html]
http://www.ftimeters.com/tech_library/flowmeter_viscosity.htm 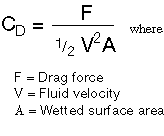 A plot of CD vs. Re usually looks
something like Figure 6. The drag coefficient decreases rapidly with the
Reynolds Number in laminar flow, rises abruptly in the transition region, then
levels off and eventually decreases slowly in the turbulent region. http://wiki.xtronics.com/index.php/Viscosity A sticky subject Laminar Flow So now how do we measure this resistance? Friction is a force (in Newtons) acting against the direction of travel. This frictional force scales with the surface area in (in m2). This friction is also inversely related to the thickness of the tar (in m). We all know that it is easier to stir molasses slowly so we know that the force goes up with the speed of stirring (in m/S). With Pl representing the our constant of viscosity we derive it here: Force = Pl x (Area of plate) x (speed) / (thickness) or: Pl = Nms/m3 = Ns/m2 This is viscosity; a unit of power per unit of area, unit of speed and unit of speed gradient. (see this) The Abbreviation Pl is the SI (mks) unit is called a Poiseuille. Unfortunately, no one uses it; instead they use the old cgs version of this formula expressed in dyne-seconds per cm2 which is called a Poise (it is smaller, thus shorter (;-). Actually, industry uses the centa-Poise or one-hundredth of a Poise because water has a viscosity of 1.002cP which is very close to 1. So one Poiseuille = Ns/m2 = Pa*s = 10 aPoise = 1000 cP Jean Louis Poiseuille (1799 - 1869) Poiseuille’s interest in the forces that affected the blood flow in small
blood vessels caused him to perform meticulous tests on the resistance of flow
of liquids through capillary tubes. In 1846, he published a paper on his
experimental research. Using compressed air, Poiseuille forced water (instead
of blood due to the lack of anti-coagulants) through capillary tubes. Because
he controlled the applied pressure and the diameter of the tubes, Poiseuille’s
measurement of the amount of fluid flowing showed there was a relationship. He
discovered that the rate of flow through a tube increases proportionately to the
pressure applied and to the fourth power of the tube diameter. Failing to find
the constant of proportionality, that work was left to two other scientists, who
later found it to be
(This story is somewhat misleading because blood flow pulsates and the viscosity of blood declines in capillaries as the cells line up single file. Thus, even with this information we have to wait for the discovery of anticoagulants - (by a long hair, Karl Paul Link, at University of Wisconsin -- vitamin K) to do definitive experiments.) Poiseuille's Law of The Flow of Liquids Through a Tube: Where:
Then:
While the viscosity of solids and liquids falls with increasing temperature (inverse relationship), the viscosity of a gas increases. While it seems counter intuitive, the rise in gas viscosity as a function temperature can be understood. As a gas gets higher in temperature it has more collisions, and thus, more friction with its neighboring molecules. The dependence on pressure of Viscosity So water and gas's viscosity are mostly independent to pressure. With Gases until the pressure is less than 3% of normal air pressure the change is negligible on falling bodies. The Poiseuille and the Poise are units of dynamic
viscosity sometimes called absolute viscosity.
If there are two numbers listed above it refers to two difference sources of
the information. None of the source measurements had any error number associated
with them. A reader enlightens me with this interesting twist A kind reader noticed (from an older version of this page) my failed attempt at explain viscosity (I tried to make the equation to mean a unit of power per unit of area - something it is not). My goal was to put viscosity in to an intuitive concept using an energetic approach to grasping the units. My Idea was that viscosity could be seen as the ability to absorb power. Thanks to our reader- in his own words which furthers the analogy
Was this Information Useful?If you found this information useful - all I ask is to look at our home page and see if we have any products that might be of use to you or a colleague. Links are Appreciated . If you have something to add to this page please send it to the e-mail below.
(C) Copyright 1994-2007, Transtronics, Inc. All rights reserved |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||